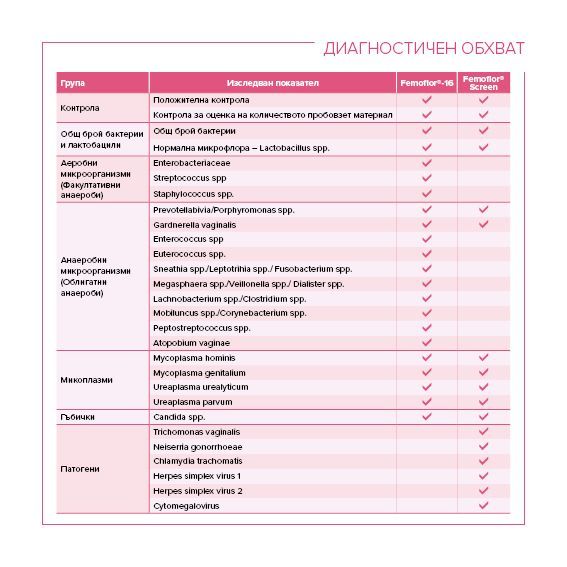
Femoflor ®
Femoflor ® is a modern PCR-based test and the only diagnostic method that allows complex evaluation of vaginal microbiome composition, diagnosis of dysbiosis and detection of sexually transmitted infections. It is a first that with just one test is possible to determine the microbial vaginal composition in relation to all microorganisms inhabiting the female vaginal tract. Femoflor ® is the only test showing the full picture of the vaginal bacterial composition, distinguishing the norm from the disease. Bacterial vaginosis (BV) is a condition where certain bacteria predominate in the vagina. BV is associated with an imbalance of „good“ and „harmful“ bacteria, usually found in a woman's vagina. Bacterial vaginosis is the most common vaginal condition in women aged 15-44 years. Bacterial vaginosis is very often asymptomatic and can increase the risk of contracting sexually transmitted infections, as well as compromising intimate and reproductive health. Pregnant women are at increased risk of developing BV. Bacterial vaginosis during pregnancy is associated with a risk of miscarriage, premature birth, low birth weight and infection of the newborn. Treatment is especially important for pregnant women.
Diagnostic range: Lactobacillus spp., Enterobacterium spp., Streptococcus spp., Staphylococcus spp., Gardnerella vaginalis, Prevotella bivia, Porphyromonas spp, Eubacterium spp, Sneathia spp., Leptotrichia spp., Megasphaera spp., Veillonella spp., Dialister spp., Lachnobacterium spp., Mobiluncus spp., Corynebacterium spp., Clostridium spp., Peptostreptococcus spp., Atopobium vaginae, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, Candida spp. , Mycoplasma genitalium, Fusobacterium spp
Diagnostic Method: Real-time PCR assay
Research Material: Vaginal swab
Results within: 1 business day
Fee: BGN 125
Clinical application
Diseases associated with bacterial vaginosis
Download Our Brochure
Mycoplasmas / Ureaplasmas / Chlamydia
Chlamydia is a common STD that can infect both men and women. It can cause serious, permanent damage to a woman's reproductive system. This can make it difficult or impossible for her to get pregnant later on. Currently, PCR assay is the gold standard for the diagnosis of chlamydial infections (See the diagnostic recommendations of the Centers for Disease Control and Prevention HERE).
In men, genital mycoplasmas are responsible for approximately 15% –20% of cases of non-gonococcal urethritis (NGU), 20% –25% of non-chlamydial non-gonococcal urethritis and approximately 30% of persistent or recurrent urethritis. In women, genital mycoplasmas are associated with the development of bacterial vaginosis and are found in 10% -30% of women with clinical cervicitis. Genital mycoplasmas are found in up to 22% of cases of pelvic inflammatory disease (PID). It demonstrates the involvement of these bacteria in the development of reproductive problems - infertility, miscarriages, premature birth, infection of the newborn. Genital mycoplasmas are highly unstable in the external environment. Microbiological culture fails to detect 40-50% of gynecological samples. For Mycoplasma genitalium culture can take up to 6 weeks and only a few laboratories in the world are able to restore clinical isolates. Therefore, PCR is the preferred method for detecting genital mycoplasmas.
Diagnostic Range: Chlamydia trachomatis/ Mycoplasma hominis/ Mycoplasma genitalium/ Ureaplasma urealiticum/ Ureaplasma parvum
Diagnostic Method: PCR
Research Material: Cervical sample / Urine / Ejaculate / Endometrial menstrual tissue
Results within: 1 business day
Fee: BGN 77
Clinical application
Vaginitis; Cervicitis; Urethritis; Epididymitis; Prostatitis; Premature birth; Pelvic inflammatory disease; Ectopic pregnancy, Infertility; Spontaneous abortions; Newborn infections
Download Brochure
Femoflor Plus®
Femoflor plus® is a modern PCR-based test and the only diagnostic method that allows complex evaluation of vaginal microbiome composition, diagnosis of dysbiosis and detection of sexually transmitted infections. It is a first that with just one test is possible to determine the microbial vaginal composition in relation to all microorganisms inhabiting the female vaginal tract. Femoflor ® is the only test showing the full picture of the vaginal bacterial composition, distinguishing the norm from the disease.
Diagnostic Range: Lactobacillus spp., Enterobacterium spp., Streptococcus spp., Staphylococcus spp., Gardnerella vaginalis, Prevotella bivia, Porphyromonas spp, Eubacterium spp, Sneathia spp., Leptotrichia spp., Megasphaera spp., Veillonella spp., Dialister spp., Lachnobacterium spp., Mobiluncus spp., Corynebacterium spp., Clostridium spp., Peptostreptococcus spp., Atopobium vaginae, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, Candida spp. , Mycoplasma genitalium, Fusobacterium spp, Chlamydia trachomatis, Trichomonas vaginalis, Neisseria gonorrhoeae
Diagnostic Method: Real-time PCR assay
Research Material: Vaginal and cervical specimen
Results within: 1 business day
Fee: BGN 187
Diseases associated with the range of Femoflor plus®
Vaginitis; Cervicitis; Predisposition to sexually transmitted infections; Pelvic inflammatory disease; Infertility; Spontaneous abortions; Premature birth; Neonatal infection
Download Our Brochure
Download Our Brochure
Chlamydia + Gonorrhea
Chlamydia is a common STD that can infect both men and women. It can cause serious, permanent damage to a woman's reproductive system. This can make it difficult or impossible for her to get pregnant later on. Currently, PCR assay is the gold standard for the diagnosis of chlamydial infections (See the diagnostic recommendations of the Centers for Disease Control and Prevention HERE).
Gonorrhea is a sexually transmitted disease caused by an infection with the bacterium Neisseria gonorrhoeae. It can infect both men and women, and can be contracted through vaginal, anal, or oral sex with someone who already has gonorrhea. This is a very common infection, especially among young people aged 15-24. It can affect the genitals, rectum, and throat, depending on sexual practices. Gonorrhea can cause very serious complications when left untreated. A pregnant woman with gonorrhea can pass the infection to her baby during delivery. Currently, PCR analysis is the gold standard for the diagnosis of gonococcal infections (See the diagnostic recommendations of the Centers for Disease Control and Prevention HERE).
Diagnostic Range: Chlamydia trachomatis/Neisseria gonorrhoeae
Diagnostic Method: PCR Assay
Research Material: Cervical specimen / Menstrual tissue / Urine / Ejaculate
Results within: 1 day
Fee: BGN 55
Clinical application
Cervicitis; Urethritis; Epididymitis; Prostatitis; Premature birth; Pelvic inflammatory disease; Ectopic pregnancy, Infertility; Spontaneous abortions; Newborn infections
Download Our Brochure
Androflor®
Androflor® is the only complex test for determining the clinically significant concentration of all microorganisms inhabiting the male urogenital tract. Infections in the male urogenital tract are a global problem and are gaining ever more social and economic significance.
Urogenital infections are the most common cause of reproductive dysfunction in men and a major factor in infertility development. Acute and chronic infections damage the germinal epithelium of the testicles, disrupt spermatogenesis, and impair the quality of semen. The lack of symptoms is the reason infections go unnoticed and transmitted to the sexual partner.
Diagnostic Range: ACE
Diagnostic Method: Real-time PCR assay
Research Material: Urine / Ejaculate
Results within: 1 business day
Fee: BGN 197
Diseases associated with the range of Androflor®
Urethritis; Enlarged prostate; Acute prostatitis; Chronic prostatitis; Orchitis and epididymitis; Balanitis; Inflammation of the seminal ducts; Inflammation of the spermatic cord, tunica vaginalis and vas deferens; Secondary infertility
Download Our Brochure
Trichomoniasis / Candida / Gardnerella
Trichomoniasis is a very common sexually transmitted disease (STD). It is caused by infection with a protozoan parasite called Trichomonas vaginalis. Although symptoms of the disease vary, most people who have the parasite cannot tell they are infected. Only about 30% develop any symptoms of trichomoniasis.
The parasite passes from an infected person to an uninfected person during sex. In women, the most commonly infected part of the body is the lower genital tract (vulva, vagina, cervix, or urethra). In men, the most commonly infected body part is the inside of the penis (urethra). Pregnant women with trichomoniasis are more likely to have their babies too early (preterm delivery).
It is not possible to diagnose trichomoniasis based on symptoms alone. For both men and women, the health care provider can direct a laboratory test to diagnose trichomoniasis.
Candida and Garnerella are among the most common causes of bacterial vaginosis. Bacterial Vaginosis (BV) is a condition that happens when there is too much of certain bacteria in the vagina. BV is linked to an imbalance of “good” and “harmful” bacteria that are normally found in a woman’s vagina. Bacterial vaginosis is the most common vaginal condition in women ages 15-44. Many women with BV do not have symptoms. If left untreated BV can increase the risk of getting other STDs and compromise the intimate and reproductive health.
Pregnant women are at increased risk of developing BV. Bacterial vaginosis during pregnancy is associated with a risk of miscarriage, premature birth, low birth weight and infection of the newborn. Treatment is especially important for pregnant women.
Diagnostic Range: Trichomonas vaginalis/ Candida albicans/ Gardnerella vaginalis
Diagnostic Method: PCR Assay
Research Material: Vaginal specimen
Results within: 1 business day
Fee: BGN 77
Clinical application
Vaginitis; Predisposition to sexually transmitted infections; Pelvic inflammatory disease; Infertility; Spontaneous abortions; Premature birth; Neonatal infection
Download Our Certificate
HPV + ThinPrep® PAP Test
The smear test (PAP test) is a medical test used in prevention of cervical cancer. There are 2 options for cytological screening for cervical cancer: conventional cytology, known as cytosmear and liquid-based cytology - a new generation cytosmear. The latter stands out for its ability for earlier, accurate and definitive diagnosing and is used as a major diagnostic platform in all developed countries.
The only method for diagnosing HPV is to perform PCR assay, where DNA fragments of the virus are isolated and amplified. The HPV genotyping test has extremely high specificity and sensitivity. It is the earliest prognostic marker for cervical cancer (the second most common cancer after breast cancer among women). The test detects 12 oncogenic types associated with a high risk of developing cervical cancer. It distinguishes different virus types, and the result is a specific conclusion (positive / negative) for each HPV type.
Diagnostic Range: Gynecological cytology; HPV 16, 18, 12 HPV hrHPV
Diagnostic Method: PCR assay; Liquid-based cytology
Research Material: Cervical specimen in a ThinPrep® container
Results within: 1-5 business days
Fee: BGN 125
Clinical application
Cervical dysplasia; Cervical cancer
Download Our Brochure
Download Our Brochure
COVID-19 + Influenza A and Influenza B (PCR Test)
This test offers simultaneous testing for COVID-19, influenza A and influenza B with a single sample of nasopharyngeal or oropharyngeal specimen (nasal or throat sample). The technology is the same as in the RT-PCR tests for COVID-19. It differs in that there are several different primer and fluorescent probes specifically targeted at: influenza virus matrix protein type A (Flu A), influenza virus type B matrix protein (Flu B), and nucleocapsid gene N of SARS-CoV-2 virus.
This type of test is recommended by the WHO for the specific diagnosis and differentiation of the three viruses as causes of acute respiratory disorders with similar symptoms but with different causes and different treatments.
Diagnostic Range: SARS-CoV-2; Influenza A; Influenza B
Diagnostic Method: RT-PCR Assay
Research Material: Nasopharyngeal and oropharyngeal specimens
Results within: For samples taken before 1.00 p.m. - the same day. For samples taken after 1.00 p.m. – before 2.00 p.m. the next business day.
Fee: BGN 135
Clinical application
SARS-CoV-2; Influenza A; Influenza B
COVID-19 + Influenza A and Influenza B + RSV (PCR Test)
This test offers simultaneous testing for COVID-19, influenza A, influenza B and respiratory syncytial virus (RSV) with a single sample of nasopharyngeal or oropharyngeal specimen (nasal or throat sample). The technology is the same as in the RT-PCR tests for COVID-19. It differs in that there are several different primer and fluorescent probes specifically targeted at: influenza virus matrix protein type A (Flu A), influenza virus type B matrix protein (Flu B), RSV and nucleocapsid gene N of SARS-CoV-2 virus.
These types of tests are recommended by the WHO for the specific diagnosis and differentiation of the four viruses as causes of acute respiratory disorders with similar symptoms but with different causes and different treatments.
Diagnostic Range: SARS-CoV-2; Influenza A; Influenza B; RSV
Diagnostic Method: RT-PCR Assay
Research Material: Nasopharyngeal and oropharyngeal specimens
Results within: For samples taken before 1.00 p.m. - the same day. For samples taken after 1.00 p.m. – before 2.00 p.m. the next business day.
Fee: BGN 140
Clinical application
COVID-19, influenza A and influenza B, non-influenza acute respiratory infections
ARVI Screen 18®
PCR test for simultaneous detection of 18 pathogens associated with acute respiratory viral infections. It includes testing for the new coronavirus, the two influenza and 15 non-influenza viruses.
The test detects a number of respiratory viruses associated with influenza and non-influenza respiratory infections, distinguishing them from SARS-CoV-2, that causes COVID-19. In addition to SARS-CoV-2, influenza virus type B and three separate strains of influenza virus type A, the test detects four other coronaviruses, human metapneumovirus, human rhinovirus, adenovirus, bocavirus, parainfluenza virus, respiratory syncytial virus (RSV).
The test results provide information to the physician about the type of pathogen that causes the respiratory disease (viral, bacterial, or mixed etiology). This allows for an informed assessment of the therapeutic approach for the patient - for example, the need for anti-influenza drugs, antibiotics or only symptomatic treatment and more.
Diagnostic Range: SARS-CoV-2; Influenza A; Influenza B; Human Respiratory Syncytial virus (hRSv); Human Metapneumovirus (hMpv); Human Parainfluenza virus-1-4 (hPiv); ОС43, Е229, NL63, HKUI human Coronavirus (hCov); Human Rhinovirus (hRv); Human B, C, E Adenovirus (hAdv); Human Bocavirus (hBov)
Diagnostic Method: PCR Assay
Research Material: Nasopharyngeal and oropharyngeal specimens
Results within: Up to 24 hours
Fee: BGN 140
ARVI Screen 22®
This is PCR test for simultaneous detection of 22 pathogens associated with acute respiratory viral infections. It includes testing for the new coronavirus, the two influenza and 19 non-influenza viruses, covering all possible respiratory viral pathogens.
ARVI screen 22® detects a number of respiratory viruses associated with influenza and non-influenza respiratory infections, distinguishing them from SARS-CoV-2, that causes COVID-19. In addition to SARS-CoV-2, influenza virus type B and three separate strains of influenza virus type A, the test detects four other coronaviruses, human metapneumovirus, human rhinovirus, adenovirus, bocavirus, parainfluenza virus, respiratory syncytial virus (RSV), parechovirus and paramyxovirus.
The test results provide information to the physician about the type of pathogen that causes the respiratory disease (viral, bacterial, or mixed etiology). This allows for an informed assessment of the therapeutic approach for the patient - for example, the need for anti-influenza drugs, antibiotics, or only symptomatic treatment and more.
Diagnostic Range: SARS-CoV-2; Influenza A; Influenza B; Human Respiratory Syncytial virus (hRSv); Human Metapneumovirus (hMpv); Human Parainfluenza virus-1-4 (hPiv); ОС43, Е229, NL63, HKUI human; Coronavirus (hCov); Human Rhinovirus (hRv); Human B, C, E Adenovirus (hAdv); Human Bocavirus (hBov); Enterovirus (EV); Parechovirus (HPeV); Paramyxoviridae (HPIV)
Diagnostic Method: PCR Assay
Research Material: Nasopharyngeal and oropharyngeal specimens
Results within: Up to 48 hours
Fee: BGN 190
HLA-B Genotyping *
The HLA-B genotyping test is assigned as part of a set of tests to confirm or rule out a presumptive clinical diagnosis of Behcet's disease, Ankylosing Spondylitis (Bechterew's disease), Stevens-Johnson syndrome and other autoimmune diseases.
The test cannot be used alone and is usually prescribed if symptoms are already present. In these circumstances, it provides additional information, increasing or decreasing the likelihood of the patient having the disease.
Diagnostic Range: HLA-B clinically significant alleles
Diagnostic Method: Sanger sequencing
Research Material: 6 ml venous blood
Results within: 5-10 business fays
Fee: BGN 180
Clinical application
Behcet's disease; Bechterew's disease; Stevens-Johnson syndrome; Rheumatology, Immunology, Neurology; Hematology
MyHappySkin
The MyHappySkin Panel is a full package of genetic markers, divided into 5 categories, which can be analyzed separately.
1. Collagen synthesis, skin elasticity and risk of cellulite
2. Sunlight sensitivity and skin tendency to hyperpigmentation
3. Risk of inflammation and redness of the skin
4. Degree of glycation and hydration
5. Detoxification capacity and oxidative stress
The test result assists the dermatologist in creating a personalized approach to proper care of your skin.
Diagnostic Range: Dermatological problems; Premature skin aging
Diagnostic Method: Sanger sequencing/ RFLP
Research Material: 6 ml venous blood
Results within: 5-10 business days
Fee: BGN 900
Clinical application
Dermatology; Skin care